Compliance
Basic Principles
We have established a department to promote and support compliance within each Group company and developed the necessary internal systems, and rules and regulations. The structure we have created facilitates the prompt handling of cases, the formulation of measures to prevent violations, and other measures. This means that officers and employees can engage in corporate activities with peace of mind. To increase awareness of compliance of employees of the Group, we are conducting compliance education for each job level while working to grasp the level of employee awareness through questionnaire surveys and identify points for improvement.
In FY2021, we conducted interviews with each department of the Group based on the results of the compliance awareness survey and worked for more thorough compliance by gaining a greater understanding of the current situation, identifying problems, and conducting training and problem solving based on actual conditions. The Group Compliance Promotion Committee has been established to supervise the Group’s efforts toward recognizing and responding to risks that could have a significant impact on Group management and promoting a compliance system that thoroughly complies with laws and corporate ethics.
Group Compliance Promotion Structure
The Group Compliance Promotion Committee has been established to supervise the Group’s efforts toward recognizing and responding to risks that could have a significant impact on Group management and promoting a compliance system that thoroughly complies with laws and corporate ethics.
The Group Compliance Supervisor and the Group Compliance Promotion Supervisor are appointed from among the representative directors, executive directors, and directors (executive officers), respectively, who are appointed within each company and notified to the Supervisor.
Charter of Corporate Behavior/Compliance Code of Conduct
>All officer[s and employees of the Group strive to comply with the Compliance Program, which includes the Charter of Corporate Behavior, the Compliance Code of Conduct, and the Whistleblower System.
Charter of Corporate Behavior
Sustainability / Ethics and Compliance
Compliance Education and Training
We have established a department to promote and support compliance within each Group company and developed the necessary internal systems, rules, and regulations. The structure we have created facilitates the prompt handling of cases, the formulation of measures to prevent violations, and other measures. This means that Board Members and employees can engage in corporate activities with peace of mind. To increase awareness of compliance of employees of the Group, we are conducting compliance education for each job level while working to grasp the level of employee awareness through questionnaire surveys and to identify points for improvement. In FY2022, we worked to further ensure compliance by conducting training and problem solving based on actual situations upon our understanding of the current situation and by identifying problems from the results of the compliance awareness survey. The Group Compliance Promotion Committee has been established to supervise the Group's efforts toward recognizing and responding to risks that could have a significant impact on Group management and promoting a compliance system that thoroughly complies with laws and corporate ethics.
| Fiscal Year |
FY2020 |
FY2021 |
FY2022 |
| Number of compliance training held |
12 |
16 |
15 |
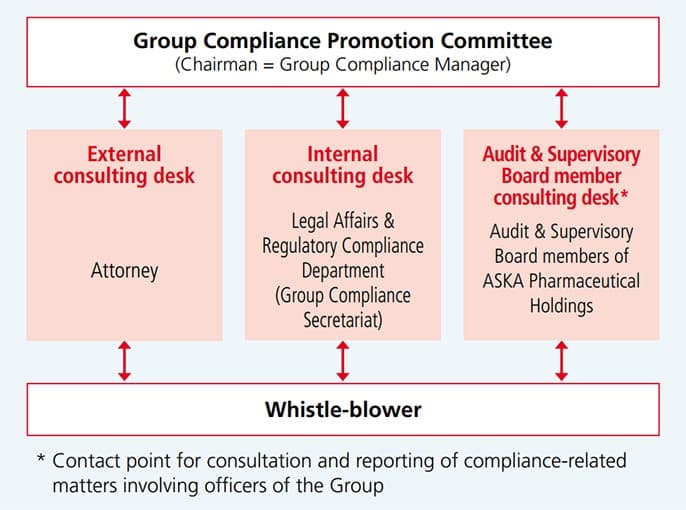
Whistle-blower System
External Consulting Desk
The Company has established a contact point for external whistleblowers. We maintain a contact point for inquiries in the event of any perceived violation of laws, regulations, or ethics in our dealings with the Company.
Consultation and Inquiries about Compliance and Human Rights
Internal Consulting Desk
As a system to reflect the voices of employees in compliance practice, we have established a whistleblower office (the ASKA Pharmaceutical Holdings’ compliance consulting desk) and have promoted awareness of the desk through education and training programs.
In addition to the existing internal and external contact points, we have established a new contact point staffed by ASKA Pharmaceutical Holdings’ Audit & Supervisory Board members (the Audit & Supervisory Board member consulting desk) for consultation and reporting of compliance-related matters involving Board Members of the Group (in connection with the enforcement of the revised Whistleblower Protection Act on June 1, 2022). This contact point, which is independent from senior management, is intended to lower the psychological hurdle for Board Members and employees for consultation and reporting of compliance-related matters involving senior management, and to ensure that investigations and corrective measures are carried out in a timely and appropriate manner. Reporting can be also done anonymously, and all cases are handled appropriately.
In addition, we have enhanced the whistleblower system by strengthening the external contact point, including increasing the number of personnel in charge (corporate lawyer’s office). In accordance with the spirit of the Whistleblower Protection Act, we operate this system to protect whistleblowers as well as to ensure that compliance-related matters are reported, investigated, and corrected in a timely and appropriate manner.
Anti-Bribery and Corruption Policy
ASKA Pharmaceutical Holdings Group (the "Group") is committed to preventing bribery and corruption in accordance with the Charter of Corporate Behavior and the Code of Conduct for Compliance.
This policy applies to all officers and employees of the Group. We request the same understanding from all domestic and overseas stakeholders, including those in our supply chain, who are involved in our business activities.
Prohibition of Bribery
In all of its business activities, whether in Japan or overseas, the Group shall engage in fair, transparent, and free competition, and shall maintain sound and normal relationships with medical professionals, related business partners, government agencies, political organizations (individuals), and others. We prohibit the giving or receiving of illegal money or other forms of payment.
Prohibition of Bribery by Business Partners, Agents, etc.
We request that all domestic and overseas stakeholders, including those in our supply chain, involved in our business activities understand the prohibition of bribery.
Anti-Bribery Due Diligence
The Group conducts due diligence on companies with which it intends to enter into new transactions or with which it already does business in order to mitigate risks related to bribery and corrupt practices.
Prohibition of Illegal Political Contributions
The Group prohibits illegal political contributions and other corrupt practices.
Entertainment and Gifts in Compliance with Laws and Internal Standards
When providing or receiving entertainment or gifts with business partners, our Group conducts corporate activities in compliance with applicable laws and internal standards. In cases of uncertainty, employees are required to consult with the responsible department in advance.
Prohibition of Anti-Competitive Practices
Our Group is committed to ensuring fair market competition and eliminating unfair trade practices. We strictly prohibit anti-competitive behavior in accordance with relevant laws and social norms.
Laws
- Antimonopoly Act
- Unfair Competition Prevention Act
- Act against Unjustifiable Premiums and Misleading Representations
- Subcontract Act, etc.
Social Norms
- Fair Competition Code
- JPMA Charter of Corporate Behavior
- JPMA Compliance Program Guidelines
- JPMA Code of Practice
- Transparency Guidelines for the Relation between Corporate Activities and Medical Institutions, etc.
- Code of Practice for Prescription Drug Promotion
- Guidelines on Collaboration with Patient Organizations, etc.
Code of Practice
As a responsible life science company handling pharmaceuticals, we recognize the need to ensure a high level of ethics and transparency in all our corporate activities. Based on this recognition, we have established the “Aska Pharmaceutical Code of Practice” as a standard of conduct governing interactions with all officers and employees, as well as with researchers, healthcare professionals, and patient organizations. By thoroughly communicating and enforcing this code among our officers and employees, we promote corporate activities that earn the trust and understanding of society.
Role of Top Management and Enforcement of this Policy
The top executives and other officers shall take the initiative in taking action to prevent bribery and corruption in accordance with the Charter of Corporate Behavior and the Code of Conduct for Compliance, and shall ensure that all employees are fully aware of these standards.
In addition, we will understand internal and external opinions, build effective governance, and ensure that corporate ethics are thoroughly upheld. In the event of a violation of this policy, top management will take responsibility for resolving the problem, investigating the cause, preventing recurrence, and promptly and accurately disclosing information to the public. In addition, the top management will fulfill its responsibility as a corporation, restore trust in the Company, and take strict disciplinary action, including that of the Board of Directors and officers.
Protection of Intellectual property
Intellectual property rights include patent rights, utility model rights, design rights, and trademark rights, which protect creations through intellectual creative activities. We respect the rights of third parties and cooperate with relevant departments to formalize and protect intellectual property rights throughout the Company. In addition to protecting rights related to newly developed products, we maximize value by utilizing intellectual property rights to extend the life cycle of existing products, including new dosage formulations. Moreover, we are promoting the globalization of our intellectual property activities and have established a system that enables us to collect and utilize intellectual property information overseas. We are working to strengthen cooperation with our partner companies in terms of intellectual property. With the growing importance of intellectual property, we will further contribute to our business growth through these efforts.
Intellectual Property Strategy
Risk Management
Basic Policy on Risk Management
In order to deal with risks that may affect the business activities of the Group, we have established the Group Business Risk Management Rules and are implementing a risk management system with classifications based on characteristics and risks. Each department prepares and operates procedure manuals and systematically works to resolve issues through annual risk assessments and the formulation, implementation, and evaluation of countermeasures based on the results of those assessments.
Risk Management System
Every organization within the Group has formulated and
implemented a business risk management manual in a bid to
avoid risks and minimize damage. If crises actually occur, the
Task Force takes action as necessary.
Business Continuity Plan(BCP)