We are committed to manufacturing and supplying safe and secure pharmaceutical products to ensure that its products do not cause any disadvantage to consumers or any negative impact on society.
Our Approach and Attitude toward Customers
1. Providing Safe and Reliable Products and Information
- We provide safe and reliable products and services through strict quality control and safety evaluations.
- We scientifically evaluate product safety information and provide information that ensures proper use.
2. Improvements Based on Dialogue with Customers
- We sincerely listen to customer feedback and respond promptly and courteously.
- Inquiries are shared and analyzed within the company, and the results are reflected in product development and support systems.
3. Building Honest and Transparent Relationships
- We strive to disclose accurate and easy-to-understand information, while adhering to fair and trustworthy standards of conduct.
- We strictly manage and protect personal information in accordance with laws and guidelines.
Basic Policy on Public Relations and Advertising
1. Accurate and Clear Communication
- Information about our products and initiatives is based on scientific evidence, and expressed with care to avoid misunderstanding or creating excessive expectations.
2. Compliance with Laws and Industry Rules
- We comply with all relevant laws and industry guidelines, including the Pharmaceuticals and Medical Devices Act (PMD Act), and conduct responsible public relations and advertising activities.
3. Promoting Honest and Trustworthy Communication
- With awareness of our corporate social responsibility, we strive to use expressions that respect human rights and diversity, and we will continue to deliver information in a reliable and trustworthy manner.
Our Approach to Safety and Security
The ASKA Group believes that protecting the quality and safety of our products is a fundamental mission that supports the foundation of our company.
Under a strict management system based on compliance with laws and scientific evidence, each employee performs their duties with a strong sense of ethics and responsibility.
We place importance on accuracy and transparency of information, and will continue to provide products that can be used with confidence, along with trustworthy information.
Internal Education and Training for Customer Response
The ASKA Group conducts various education and training programs within each department.
Customer Response and Initiatives
Research and Development
Aiming to create new drugs to meet future medical needs
ASKA Pharmaceutical's research fields focus on internal medicine, obstetrics and gynecology, and urology, with the aim of continuously and quickly discovering high-value drugs that can meet the needs of patients. By leveraging its accumulated technologies and experiences, the Company is actively engaged in research and development activities with a well-balanced structure. In particular, by organizing two divisions, the Innovative Drug Discovery Division, which is responsible for basic research and non-clinical studies, and the Development Division, which is responsible for clinical studies, the Company is consolidating functions and clarifying authority in order to improve operational efficiency.
Furthermore, the Company is expanding its development pipeline through the use of cross-departmental theme exploration projects, enhanced seed discovery through the promotion of open innovation, and active in-licensing activities. The Medical Science Liaison (MSL) team was newly established in the Development Division to provide total coordination from development to post-marketing product strategy as a healthcare specialist.
Research and Development Structure
The Drug Discovery Research Division is capable of covering all aspects of drug discovery, including the search for drug seeds, the drafting of development concepts, the synthesis of new substances, and the narrowing down of candidate compounds for development (examining efficacy, pharmacokinetics, and toxicity).
In addition to conducting research in each department, the division also organizes cross-departmental project teams for each theme. Team members work to maximize their abilities so that the results obtained can be consolidated and promptly transferred to clinical trials.
In compliance with the ethical principles of the Declaration of Helsinki and the Good Clinical Practice (GCP), the Development Division confirms the efficacy and safety of new drugs in Phase I, Phase II, Phase III, Long-term administration studies, and biological equivalence studies.
In order to deliver new drugs that match medical needs to patients as soon as possible, the Company conducts clinical trials through consultation with the Pharmaceuticals and Medical Devices Agency (PMDA) to determine the best clinical trial plan and trial structure in the shortest possible time.
Each department works together and cooperates with each other to achieve early approvals.
Providing Drug Information
It must be recognized that pharmaceutical information provision is an activity with the noble mission of accurately communicating drug information to healthcare professionals and assuming responsibility for the proper use of pharmaceuticals. Pharmaceutical companies are required not only to provide accurate information to medical professionals, but also to maintain higher ethical standards as a life-related industry.
Through a systematic training program consisting of new employee induction training and ongoing training, the Company complies with the Pharmaceuticals and Medical Devices Act, the Antimonopoly Act, the Fair Competition Code, the Promotion Code, and the Code of Practice, and conducts information activities in a fair and transparent manner. In addition, when providing information on its products to medical professionals, the Company does not deviate from the scope of its manufacturing and marketing approval and provides information that is unbiased in terms of efficacy and safety.
Customer Support System
Drug Information Unit
The Drug Information Unit receives many inquiries on a daily basis. The inquiries are diverse, ranging from those from patients who are taking ASKA Pharmaceutical's pharmaceuticals and have vague concerns to those from physicians and pharmacists inquiring about specialized pharmaceutical information. In order to respond to such inquiries, the unit members strive to acquire product knowledge and make other efforts to improve themselves on a daily basis. As an open point of contact for pharmaceutical information, the Unit is committed to contributing to the improvement of everyone's health by accurately responding to inquiries and promoting the proper use of the pharmaceuticals.
In addition, the Unit strives to ensure the appropriate management and handling of personal information that it comes into contact with when responding to phone calls, in accordance with the company's privacy policy.
Mechanisms to respond to user feedback
Our group has a system in place to reflect the valuable feedback we receive from medical professionals (patients) within the Group.
Explanations and instructions for product use
Example 1
<User's Voice>
When I am busy, I forget to take my medication. Is there any way to take them properly?
<Improvement details>
We developed a medication support app. The app is provided for free to eligible patients through medical institutions. The smartphone app, which patients use all the time, provides a reminder function to prevent patients from forgetting to take their medications.
-
Screen image of the dosing support app
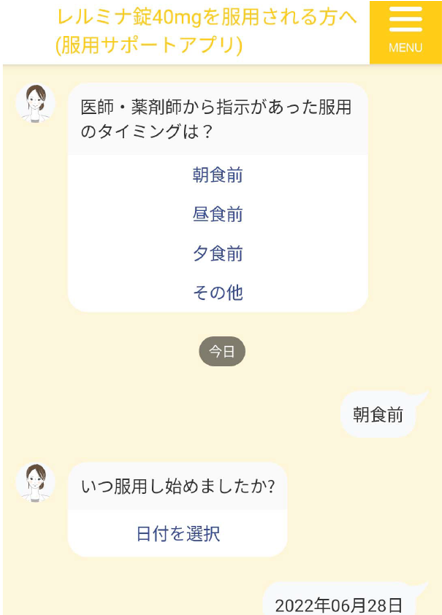
-
Dosing support app information materials

Example 2
<User's Voice>
The doctor taught me how to use the suppository, but when I tried it at home by myself, I couldn't figure out how to use it. Is there any easy way to confirm the instructions?
<Improvement details>
We have created a video to support how to insert suppositories. You can easily view the video by scanning the QR code with your smartphone. This allows those who are using suppositories for the first time to continue their treatment with peace of mind.
-
Suppository insertion support video
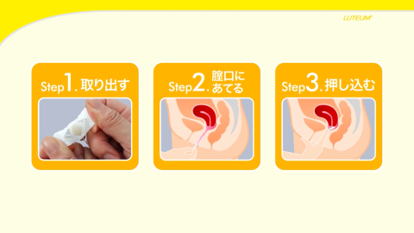
-
Material for suppository insertion support video

Example 3
<User's Voice>
Many people have the mistaken perception that menstrual pain is something to be endured, and many are unable to talk to anyone about their menstrual problems. It would be nice if they would visit a medical institution once.
<Improvement details>
We have created a disease awareness material called "If you have menstrual problems, talk to a doctor! We hope that all women will use these materials as an opportunity to have correct knowledge and to consult a doctor.
-
Disease awareness material

Production
Policy on Production Activities
Pharmaceutical companies are required to provide a reliable and stable supply of high quality pharmaceutical products.
At ASKA Pharmaceutical, all employees involved in production activities are aware of their responsibility to provide a stable supply of high quality pharmaceutical products, and conduct production activities with a high sense of ethics.
Efforts to ensure a stable supply of high-quality pharmaceutical products
ASKA Pharmaceutical continues to manufacture and supply high quality pharmaceutical products using facilities and systems that comply with GMP, a standard for manufacturing and quality control of pharmaceutical products
Through regular education and training for all employees involved in manufacturing, including quality assurance, we produce high quality pharmaceuticals and strive to ensure a stable supply of products by continuously reviewing our production facilities to ensure that production lines are appropriately expanded or upgraded.
GMP Compliance Initiatives
1. GMP Compliance System
We evaluate and confirm semiannually that GMP compliance has been thoroughly implemented and that manufacturing and quality control are being properly managed. Targets and performance assessment results are regularly reported to management and communicated to all factory employees through education and training programs.
2. Regular GMP training for all factory employees
GMP training at the factory is conducted regularly for all employees based on an annual plan. In addition, each organizational unit at the manufacturing site flexibly conducts training according to the needs of actual business operations. Furthermore, at the monthly GMP Committee meetings, organizational managers share all GMP-related issues, discuss the countermeasures, and provide important information among them to employees as part of the training content.
〈Education and training for all employees at the factory〉
- Date:December 2022, June 2023
- Participants:All employees at the factory
- Duration:1 hour for each training
3. Training within the workplace for each operation
With regard to any defects or deviations that occur, education and training are thoroughly conducted for manufacturing and quality control, including reconfirmation of internal procedures and dissemination of revised items.
4. New employee induction training
New employees assigned to the factory learn the basics of GMP, PQ*2, PV*3, etc., as well as related laws and regulations through classroom training. Afterward, through on-the-job training at their assigned sites, they can work after their supervisors approve their knowledge and skills.
- *2 *3 PQ and PV are verification methods used in pharmaceutical manufacturing.PQ aims to verify and assure that the equipment used in the manufacturing process is working correctly; PV aims to verify and assure that the manufacturing process is suitable to produce the desired product.
Supply Chain Management
ASKA Pharmaceutical is taking the following measures for supply chain management both in Japan and overseas in order to ensure a stable supply of high quality pharmaceutical products.
- Continuous supply chain re-inspection
Leveraging existing internal systems, we are enhancing and updating supplier information. By centralizing and sharing this information, we are building a system that enables us always to do better business with our suppliers.
- Supplier assessment and impact visibility
Based on the aggregated information, we evaluate suppliers, visualize their impact, and clarify priorities as we work to improve issues.
- Initiatives to maintain a stable supply
To ensure a stable supply of products, we view it as important to strengthen our production system and improve productivity. ASKA Pharmaceutical manages the entire process from stable procurement of raw materials to commercialization of products, including checking the inventory status of APIs at manufacturing sites. We are also actively engaged in exchanging information with each supplier and collaborating with new suppliers to ensure the continuation of stable supply through planned and efficient production.
- For details, please see the separate page “Supply Chain”.
Supply Chain
Use of universal fonts
ASKA Pharmaceutical is committed to the development and production of pharmaceuticals that take into account their impact on society and the environment. By adopting universal fonts for pharmaceutical packaging, we strive to design products that are easier for patients and medical professionals to read and understand.
Quality & Safety Assurance
Basic Policy for Quality and Safety Assurance
The Quality & Safety Assurance Division of ASKA Pharmaceutical
is responsible for ensuring compliance with the Law on Securing
Quality, Efficacy and Safety of Products Including Pharmaceuticals
and Medical Devices and other laws and ministerial ordinances, as
well as the Good Quality Practice (GQP), and the Good Vigilance
Practice (GVP) standards, ensuring the quality and safety of our
pharmaceutical products, and supporting a stable supply.
We carry out our responsibilities based on high ethical standards and
a sense of mission so that medical professionals and patients can
use our pharmaceuticals with confidence and peace of mind.
Our basic policy is to ensure that our pharmaceuticals are
manufactured correctly, and to provide information on the
proper use of our pharmaceuticals, and as such, contribute to
medical care.
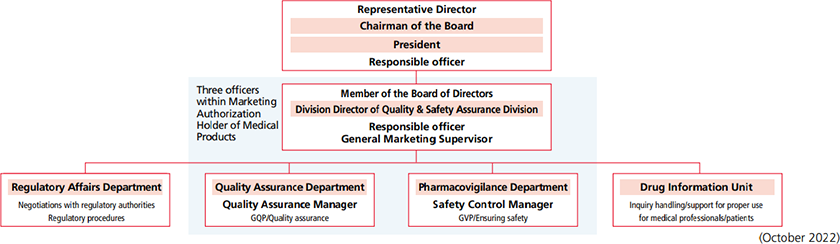
Safety Assurance System
In the Quality & Safety Assurance Division, under the supervision of
the Division Director in charge, who is also General Marketing
Supervisor and the responsible officer (an officer in charge of
operations related to pharmaceutical affairs laws and regulations),
the Quality Assurance Manager is assigned to the Quality Assurance
Department (a GQP department), and the Safety Control Manager
is assigned to the Pharmacovigilance Department (a GVP
department), while the Regulatory Affairs Department is responsible
for quality assurance and safety control of our pharmaceutical
products in a unified manner, which involves appropriately
maintaining manufacturing and marketing approval certification.
We are working to ensure compliance
with the revised Law on Securing Quality, Efficacy and Safety of
Products Including Pharmaceuticals and Medical Devices and
guidelines on legal compliance, clarify the division of duties and
scope of responsibility of responsible officers, and update and
improve Company and procedural manuals to further strengthen
our governance and other aspects of our operations. We will
continue to work closely with related divisions to ensure the reliable
provision of our pharmaceutical products to medical professionals
and patients so that they can be used with peace of mind.

For Proper Pharmaceutical Manufacturing
In order to confirm and maintain that ASKA Pharmaceutical's pharmaceutical products are properly manufactured in accordance with the manufacturing and marketing authorization, we are strengthening regular inspections of domestic and overseas manufacturing facilities and GMP audits by the Quality Assurance Department (GQP Division). In the GMP audits of the Quality Assurance Department, we are training and strengthening our auditing staff, and are considering the introduction of remote audits using web monitors, in addition to the existing on-site audits and paper-based investigations.
In the event that a deviation occurs in the manufacturing process, we will properly evaluate and analyze the situation, track down the cause, and focus on preventing recurrence through CAPA* measures.
In addition, we have completed the GS1 barcode labeling, which incorporates information such as product code, expiration date, and serial number for each packaging form, to prevent accidents due to drug mix-ups, ensure traceability, and improve distribution efficiency.
We will continue our efforts to deliver safe and reliable pharmaceutical products to medical professionals and patients.
- * CAPA:Corrective Action and Preventive Action
Initiatives of Quality and Safety Assuarance Division
Japan relies on other countries for many raw materials,
including active ingredients. Now, more than ever, we need to
strengthen our relationships with overseas manufacturing
plants and partner companies so that we can continue to
provide a stable supply of pharmaceuticals with the same quality
and safety assurance levels that we have always done. ASKA
Pharmaceutical complies with all laws and regulations and
promotes the strengthening of quality systems, safety measures,
and proper use of its products. At the same time, we maintain
close collaboration between the three officers within the
Marketing Authorization Holder of Medical Products and relevant divisions. The Quality & Safety Assurance Division undertakes
various initiatives to keep up with the latest developments
with the aim of achieving continued growth. As such, it is
refining its expertise and information-gathering capabilities,
improving its communication and problem-solving skills, as
well as its ability to operate effectively in the English language
with the aim of forming win-win relationships with overseas
manufacturing plants and building and developing organizations
and human resources trusted by all partner companies.


Education and Training for Board Members and All Employees
ASKA Pharmaceutical conducts annual educational training for Board members and all employees on compliance with laws and regulations and how to respond to hearing of an adverse event.
Regarding legal compliance, participants learn about the contents of the "Legal Compliance Guidelines," the responsibilities of responsible officers, and how to get involved as an employee. In addition, in the area of handling adverse events, e-learning is provided for employees other than MRs on how to report adverse events to the Pharmacovigilance department via the intranet when they hear about adverse events from medical professionals or in daily conversations with family members or friends.
<Actual results of educational training using e-learning>